Sir Hugh Taylor talks about the need for commitment and collaboration to make sure the Accelerated Access Review proposals become reality.
Accelerated Access Review
- Notice: Patent Journal special notices: 7066
- Guidance: Requests to amend a patent after grant
- Guidance: Offender management: caseworker guidance
- Press release: Prime Minister warns Russian threat to global stability is accelerating as Putin ramps up attacks on Black Sea
- Transparency data: Transport Research Innovation Grant (TRIG): funding winners
- Press release: Cutting-edge transport projects receive £1.4 million to encourage innovation and deliver growth
- Press release: Governments launch largest review of sector since privatisation
- Press release: Landmark UK-Germany defence agreement to strengthen our security and prosperity
- Press release: Collision of two passenger trains at Talerddig, Powys, Wales
- Guidance: Section 62A Planning Application: S62A/2024/0058 Land adjacent to Village Hall, East of Cambridge Road, Ugley, Bishops Stortford, Hertfordshire, CM22 6HR
Sets out recommendations to speed up access to innovative healthcare and technologies, to improve efficiency and outcomes for NHS patients.
New recommendations set out how patients could get quicker access to innovative new diagnostic tools, treatments, and medical technologies.
Lord Prior welcomes new ambitions to speed up patient access to innovative new treatments.
This guide gives an overview of the innovation pathway for products to be used by the NHS.
The second phase of engagement kicks off with Accelerated Access Review’s (AAR) interim report from independent Chair, Sir Hugh Taylor.
The second phase of engagement kicks off with Accelerated Access Review’s (AAR) interim report from independent Chair, Sir Hugh Taylor.
The Accelerated Access Review (AAR) has paused its online engagement to analyse the feedback received and prepare its interim report.
Join our conversation and share your views on how to speed up access to innovative drugs, devices and diagnostics for NHS patients.
A new Accelerated Access Review website is asking for views on how to improve access to transformative medicines and medical technology.
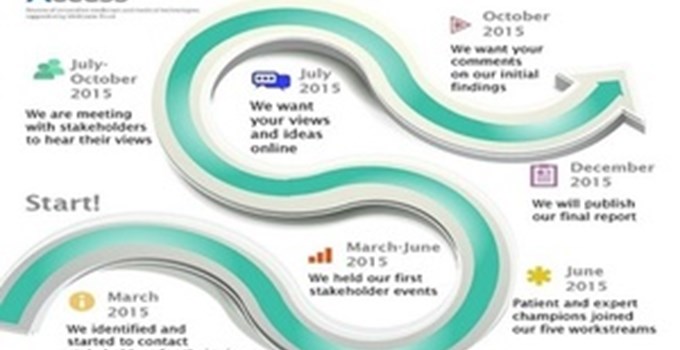
The Review has established various routes for people to give their views on quicker access to transformative medicines and medical technology.
Health and industry experts attend workshop on how to give patients better access to transformative new medicines and medical technologies.
Getting new drugs and medtech to patients faster: find out what was said.
Review is examining pathways for pharmaceuticals, medical technology and digital healthcare across four big interconnecting themes.
Help us to speed up access to innovative drugs, devices and diagnostics for NHS patients.
Ministerial Departmental News
- PM's Office, 10 Downing Street
- Cabinet Office
- Department for Business, Innovation and Skills
- Department for Communities and Local Government
- Department for Culture, Media and Sport
- Department for Education
- Department for Environment, Food and Rural Affairs
- Department for International Development
- Department for Transport
- Department for Work and Pensions
- Department of Energy and Climate Change
- Department of Health
- Foreign and Commonwealth Office
- HM Treasury
- Home Office
- Ministry of Defence
- Ministry of Justice
- Northern Ireland Office
- Scotland Office
- Wales Office
- See all departments