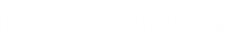Veterinary Medicines Directorate
450 articles
Showing 31-60
Product defect recall alert for four products by Dechra Pharmaceuticals PLC
Tuesday, 21 October 2025
Wholesaler level recall alert for four products by Dechra Pharmaceuticals PLC....

Small pet animal medicines exempt from authorisation: Annual submission of product list
Friday, 17 October 2025
Companies marketing medicines for small pet animals exempt from the requirements of a Marketing Authorisation must submit an annual return to the VMD ...

Tilmodil 300 mg/ml Solution for Injection for Cattle and Sheep Product defect recall
Thursday, 16 October 2025
Product recall alert for Tilmodil 300 mg/ml Solution for Injection for Cattle and Sheep by Emdoka & DUG(V) UK Limited. ...
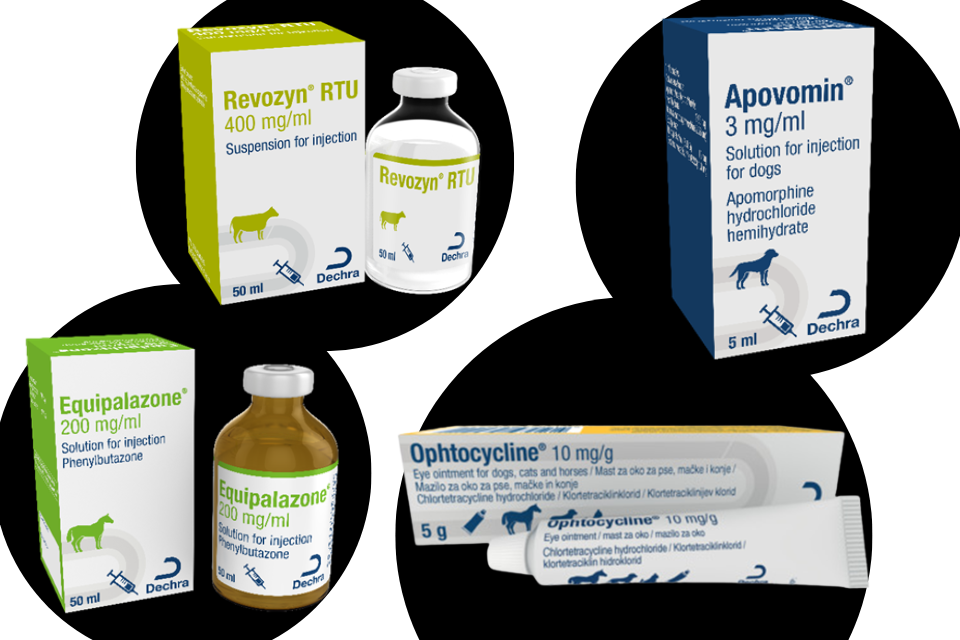
Gonavet Veyx 50 micrograms/ml Solution for Injection for Cattle, Pigs and Horses Product defect recall
Tuesday, 14 October 2025
Product recall for Gonavet Veyx 50 micrograms/ml Solution for Injection for Cattle, Pigs and Horses by Anupco Ltd T/A Kela Animal Health UK....
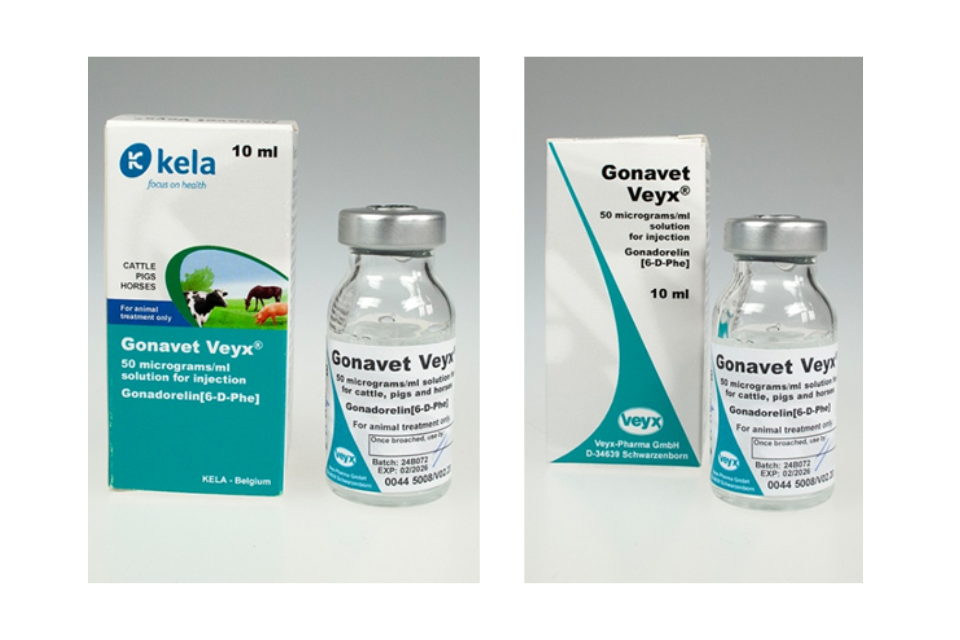
Maprelin 75 µg/ml Solution for Injection for Pigs Product defect recall
Tuesday, 14 October 2025
Product recall for Maprelin 75 µg/ml Solution for Injection for Pigs by Anupco Ltd T/A Kela Animal Health UK....

Recruitment for Higher Scientific Officer in the Biologicals Team
Tuesday, 14 October 2025
Higher Scientific Officer, working on the authorisation in the UK of biological and immunological veterinary medicinal products....

Stakeholder feedback
Monday, 06 October 2025
Our aim is to provide ways of giving feedback that are timely, targeted and accessible for all our stakeholder groups....

Recruitment for a Policy Officer
Monday, 29 September 2025
Policy officer, working on policy and legislation on veterinary medicines....

VMD launches survey to understand how pet owners use flea and tick spot on treatments
Monday, 29 September 2025
VMD survey will help inform future guidance to protect both animal health and the environment....

Ataxxa Spot on Solution for Dogs– SPC change
Friday, 26 September 2025
Change to the information provided in the Summary of Product Characteristics (SPC) for Ataxxa Spot-on Solution for Dogs....

Recruitment for a Veterinary Advisor: Antimicrobial Resistance (AMR) Surveillance & Evidence
Monday, 22 September 2025
Vet Advisor, working on matters involving AMR surveillance, research, and interventions....

Recruitment for a Safety Assessor
Monday, 15 September 2025
Safety Assessor, working on the scientific assessment of applications to vary marketing authorisations....

Government publishes framework to strengthen UK veterinary vaccine availability
Wednesday, 10 September 2025
New Statement of Intent sets out cross-sector approach to address vaccine supply challenges and support innovation in animal health....

Recruitment for an Innovation Facilitator
Wednesday, 10 September 2025
Innovation Facilitator, working to the support delivery of the VMD Science Strategy....

VMD launches call for evidence on the Medicines and Medical Devices Act as it applies to Veterinary Medicine
Sunday, 31 August 2025
The Veterinary Medicines Directorate is seeking views from stakeholders to inform future reform of the UK's veterinary medicines regulatory framework....

Recruitment for Communications and Engagement Officer
Thursday, 28 August 2025
We have a vacancy for a Communications and Engagement Officer....

DIVENCE PENTA lyophilisate and solvent for emulsion for injection – SPC change
Wednesday, 27 August 2025
Change to the information provided on adverse events in the Summary of Product Characteristics (SPC)....

Consultation: target animal safety evaluation for veterinary monoclonal antibody products
Tuesday, 19 August 2025
Invitation for comments until 15 February 2026 on a draft VICH guideline relating to target animal safety evaluation for veterinary monoclonal antibod...

VMD Strengthens Pharmacovigilance Framework Whilst Addressing Reporting Concerns
Wednesday, 13 August 2025
VMD outlines plans to strengthen pharmacovigilance framework and confirms interim adverse event reporting routes whilst a new system is developed....

Credelio 12mg and 48mg chewable tablets for cats – SPC change
Tuesday, 05 August 2025
Change to the information provided on adverse events in the Summary of Product Characteristics for Credelio 12mg and 48mg chewable tablets for cats....

Crovect 1.25% w/v Pour on Solution for Sheep Recall alert
Monday, 04 August 2025
Product defect recall alert for Crovect 1.25% w/v Pour-on Solution for Sheep, GB & NI authorisations by Elanco Animal Health....

UK launches world first study to assess antibiotic resistance levels in healthy dogs and cats
Monday, 28 July 2025
The study aims to provide key information essential for reducing the development and spread of disease resistant infections....

Government publishes plan to address presence of chemicals from pet flea and tick treatments in UK waterways
Tuesday, 22 July 2025
New plans to address the presence of chemicals from flea and tick treatments in rivers and streams across the UK have been unveiled today...

Relaunch of Introductory Veterinary Wholesale Qualified Person Training Course?
Tuesday, 22 July 2025
Online training course 11 - 12 November 2025 introducing the role and responsibilities of the Wholesale Qualified Person (WQP) and the legislative req...
Anesketin 100 mg/ml Solution for Injection for Dogs, Cats and Horses Recall alert
Thursday, 17 July 2025
Product defect recall alert for Anesketin 100 mg/ml Solution for Injection for Dogs, Cats and Horses....

StromEase 25 mg/ml Eye Drops, Solution for Dogs and Cats – SPC change
Monday, 14 July 2025
Change to the information provided on adverse events in the Summary of Product Characteristics for StromEase 25 mg/ml Eye Drops, Solution for Dogs and...

Zermex 100 mg/ml LA Solution for Injection for Cattle and Zermex 20 mg/ml LA Solution for Injection for Sheep – SPC change
Thursday, 03 July 2025
Change to the information provided on adverse events in the Summary of Product Characteristics (SPC)....

VMD relaunches Veterinary Dispensary Manager Online Course
Thursday, 03 July 2025
This popular online training event is scheduled for Thursday 23 October 2025 and is ideal for anyone involved in veterinary dispensary roles....
Further update: Genta Equine 100 mg/ml Solution for Injection for Horses – Adverse events
Friday, 27 June 2025
Actions following the identification of the cause of an increase of adverse event reports following the use of Genta-Equine 100 mg/ml Solution for Inj...

Recruitment for Biological Assessors
Tuesday, 24 June 2025
Biological Assessor, working on new marketing authorisations, variations of existing authorisations, and animal test certificates. ...