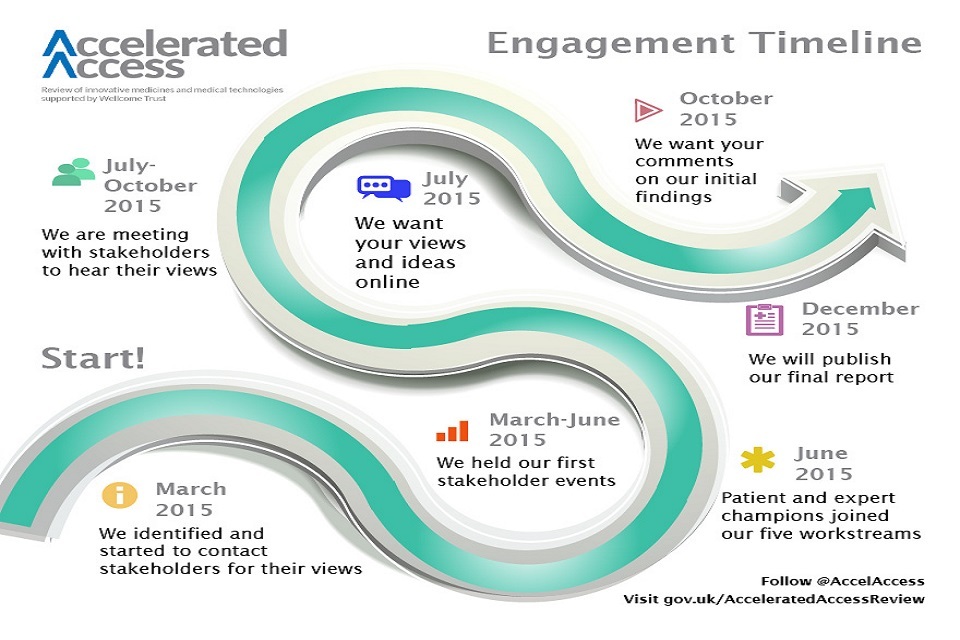
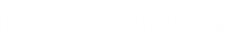As the engagement timeline below shows, the Accelerated Access Review team has been identifying, contacting and meeting with a range of people since March. More recently, we have defined various routes for contributions to us over the summer through some specific engagement activity.
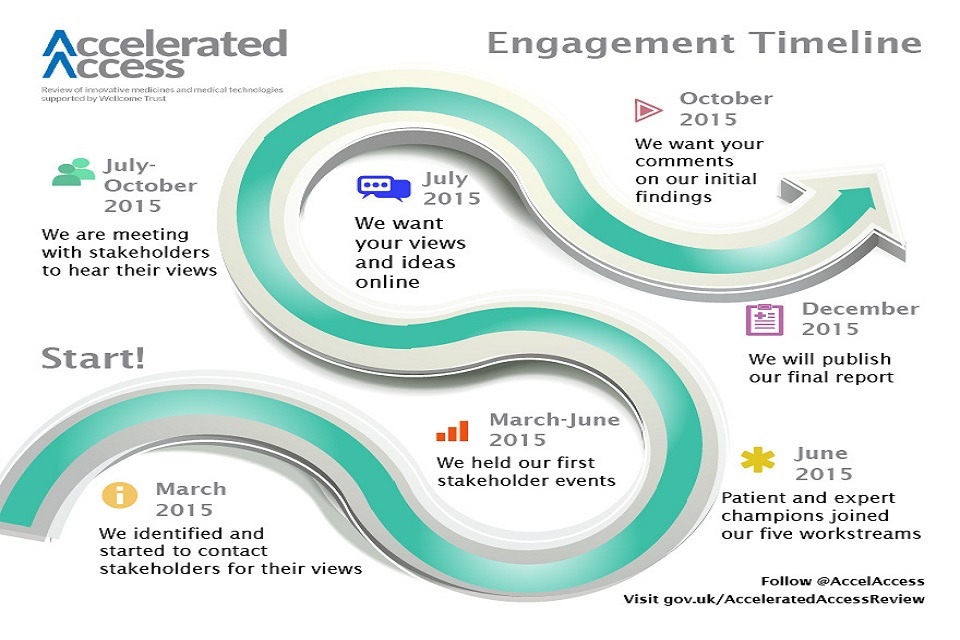
Timeline for AAR engagement activity
If you are:
- a pharmaceutical, biotech or medical technology company, get in touch with your trade association, such as the Association of the British Pharmaceutical Industry, the Association of British Healthcare Industries, the BioIndustry Association, the British In Vitro Diagnostics Association and the Ethical Medicines Industry Group
- an NHS organisation, get in touch with NHS England at england.innovation@nhs.net for further information
- a charity, National Voices are supporting the review to ensure the voluntary sector is engaged – we will publish further details shortly
Additionally, on 15 July, the Review will launch a new website which will ask a wider audience (including patients and their representatives) for their ideas about how to speed up access to transformative medicines and medical technology.