Wildlife and Animal Welfare
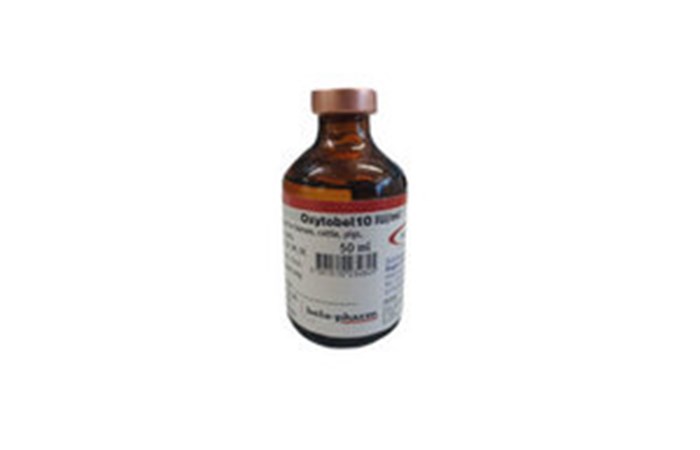
We wish to make wholesalers and veterinarians aware that Bela-Pharm GmbH & Co.KG has issued a recall of a single batch of Oxytobel 10 IU/ml Solution for Injection for Horses, Cattle, Pigs, Sheep, Goats, Dogs and Cats (Vm 41816/4000).
Bela-Pharm has identified that there is a potential for decreased levels of the preservative Chlorobutanol hemihydrate in the following batch: J2101-03 Expiry 11/2018
Oxytobel is distributed in the UK by Bimeda UK. Veterinary practices that have purchased this batch number should contact Bimeda to arrange collection.
If you have any queries in relation to return of this product or credit for the product returned please contact Kay Cowton, Bimeda UK Sales on 01248 725400 or email uksales@bimeda.com
For further information on the recall please contact Bela
