Veterinary Medicines Directorate
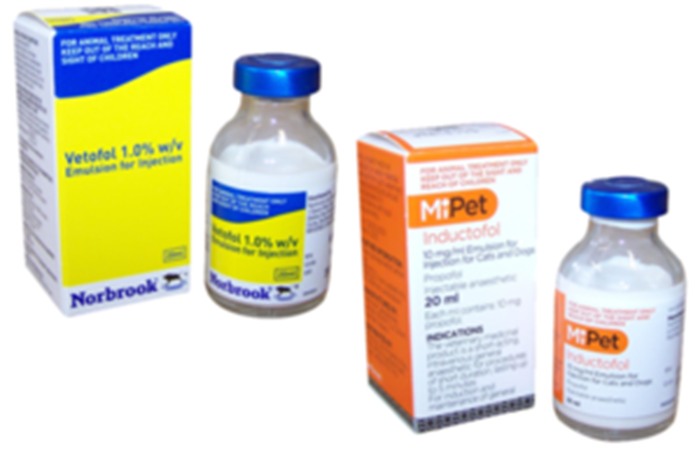
We wish to make wholesalers and veterinary surgeons aware that the VMD has been notified that Norbrook Laboratories Ltd has issued a recall of all batches of Propofol Emulsion for Injection 1.0% w/v currently on the market.
| UK Brand Name | Licence No |
|---|---|
| Inductofol | 02000/4274 |
| Vetofol | 02000/4244 |
| Norofol* | 02000/4275 |
- Not marketed in the UK
An issue of coring has been reported, where the shearing off of a portion of the 20mm bromobutyl bung occurs as the vial is pierced to withdraw the product. This may result in particles from the bung entering the product and potentially being drawn up into the syringe upon extraction of the product from the vial. It may also result in the bung not resealing fully after use.
If you have any queries in relation to the recall or require details of the product in other markets, contact Mrs Deborah Curran Tel. +4428 30264435 or email deborah.curran@norbrook.co.uk.
